
Occasionally, the atomic number is omitted in this notation because the symbol of the element itself conveys its characteristic atomic number. In this case, the mass number is 12, which means that the number of neutrons in the atom is 12 − 6 = 6 (that is, the mass number of the atom minus the number of protons in the nucleus equals the number of neurons). The mass number, the superscript next to the symbol, is the sum of the number of protons and neutrons in the nucleus of this particular isotope. Its atomic number, 6, is the subscript next to the symbol and is the number of protons in the atom. The element in this example, represented by the symbol C, is carbon. We also introduced in Chapter 4 "Atoms, Molecules, and Ions" the notation for succinctly representing an isotope of a particular atom: Protons and neutrons are located in the nucleus and provide most of the mass of an atom, while electrons circle the nucleus in shells and subshells and account for an atom’s size. We saw in Chapter 4 "Atoms, Molecules, and Ions" that atoms are composed of subatomic particles-protons, neutrons, and electrons. In this chapter, we will examine some of the basic concepts of nuclear chemistry and some of the nuclear reactions that are important in our everyday lives.ĭefine and give examples of the major types of radioactivity.
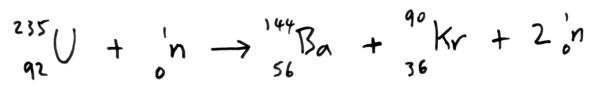
Many people are aware of nuclear power plants and nuclear bombs, but nuclear chemistry also has applications ranging from smoke detectors to medicine, from the sterilization of food to the analysis of ancient artifacts.

However, in nuclear chemistry, the composition of the nucleus and the changes that occur there are very important.Īpplications of nuclear chemistry may be more widespread than you realize. Most chemists pay little attention to the nucleus of an atom except to consider the number of protons it contains because that determines an element’s identity. Although many people have an unfounded fear of radioactivity, smoke detectors save thousands of lives every year. When the current drops beneath a set value, another circuit triggers a loud alarm, warning of the possible presence of fire.Īlthough radioactive, the americium in a smoke detector is embedded in plastic and is not harmful unless the plastic package is taken apart, which is unlikely. When particles of smoke from a fire enter the smoke detector, they interfere with the ions between the metal plates, interrupting the flow of current. (This constant drain on the battery explains why the batteries in smoke detectors should be replaced regularly, whether the alarm has been triggered or not.) The radioactivity of americium ionizes the air between the plates, causing a tiny current to constantly flow between them. Next to the plates is a small disk containing a tiny amount (∼0.0002 g) of the radioactive element americium.

A battery in the circuit creates a voltage between the plates. This device is a smoke detector.Ī typical smoke detector contains an electric circuit that includes two metal plates about 1 cm apart. Most of us have at least one device in our homes that guards our safety and, at the same time, depends on radioactivity to operate properly.


 0 kommentar(er)
0 kommentar(er)
